Identify the true statements regarding disulfide bridges disulfide bonds – Identify the true statements regarding disulfide bridges and disulfide bonds. Disulfide bridges are covalent bonds formed between two cysteine residues, playing a crucial role in protein structure and stability. Understanding their formation, disruption, and functions is essential in protein chemistry and biochemistry.
Disulfide bridges contribute to the stability and function of various proteins, including antibodies and enzymes. They influence protein folding, rigidity, and interactions with other molecules. Analytical techniques like gel electrophoresis and mass spectrometry aid in analyzing disulfide bonds, providing insights into protein structure and dynamics.
Disulfide Bridges: Identify The True Statements Regarding Disulfide Bridges Disulfide Bonds
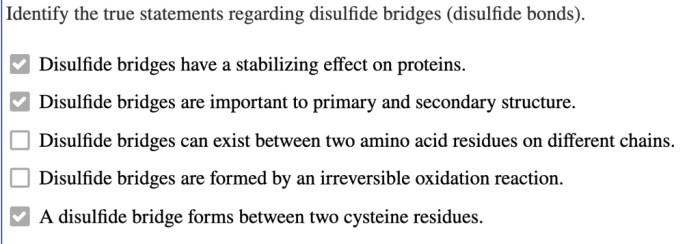
Disulfide bridges, also known as disulfide bonds, are covalent bonds that form between two cysteine residues in a protein. They play a crucial role in protein structure and stability by creating a cross-link between two polypeptide chains or within a single polypeptide chain.The
formation of disulfide bridges involves the oxidation of two cysteine residues, which contain a thiol (-SH) group. When two thiol groups react in the presence of an oxidizing agent, such as oxygen or hydrogen peroxide, they undergo a redox reaction to form a disulfide bond (-S-S-).
Formation and Disruption of Disulfide Bonds
The formation of disulfide bonds is a crucial step in protein folding and maturation. It occurs in the endoplasmic reticulum (ER) or periplasmic space of prokaryotes, where oxidizing conditions prevail. The formation of disulfide bonds is catalyzed by enzymes called protein disulfide isomerases (PDIs), which facilitate the correct pairing of cysteine residues and prevent the formation of incorrect disulfide bonds.Disulfide
bonds can be disrupted by reducing agents, such as dithiothreitol (DTT) or beta-mercaptoethanol. These agents break the disulfide bond by reducing the disulfide group (-S-S-) to two thiol groups (-SH). The disruption of disulfide bonds can lead to the unfolding or denaturation of proteins.
Properties and Functions of Disulfide Bridges, Identify the true statements regarding disulfide bridges disulfide bonds
Disulfide bridges are strong covalent bonds that contribute to the stability and rigidity of proteins. They are resistant to heat, pH changes, and proteolytic enzymes. Disulfide bridges play a crucial role in maintaining the correct conformation of proteins and preventing their aggregation.In
addition to their structural role, disulfide bridges can also participate in redox reactions. The reduction and oxidation of disulfide bonds can be used to regulate protein activity and signal transduction pathways.
Disulfide Bridges in Different Protein Contexts
Disulfide bridges are found in a wide range of proteins, including antibodies, enzymes, and structural proteins. In antibodies, disulfide bridges contribute to the stability of the antigen-binding site and the overall structure of the antibody. In enzymes, disulfide bridges can maintain the active site conformation and facilitate substrate binding.
In structural proteins, such as keratin and collagen, disulfide bridges provide strength and rigidity to the protein fibers.
Analytical Techniques for Disulfide Bond Analysis
Several analytical techniques can be used to analyze disulfide bonds in proteins. Gel electrophoresis, such as non-reducing SDS-PAGE, can be used to separate proteins based on their molecular weight and disulfide bond content. Mass spectrometry can be used to identify and characterize disulfide bonds within proteins.
Additionally, chemical methods, such as Ellman’s reagent, can be used to quantify the number of disulfide bonds in a protein sample.
FAQ Guide
What is the role of cysteine residues in disulfide bridge formation?
Cysteine residues contain a thiol group (-SH) that can oxidize to form a disulfide bond with another cysteine residue.
How do reducing agents disrupt disulfide bonds?
Reducing agents, such as dithiothreitol (DTT) or beta-mercaptoethanol (BME), donate electrons to disulfide bonds, causing their reduction and breaking.
What analytical techniques are used to analyze disulfide bonds?
Gel electrophoresis and mass spectrometry are commonly used techniques to analyze disulfide bonds, providing information on their number, location, and connectivity.